 Law Of Definite Composition 4 2 2 Introduction
Law Of Definite Composition 4 2 2 Introduction
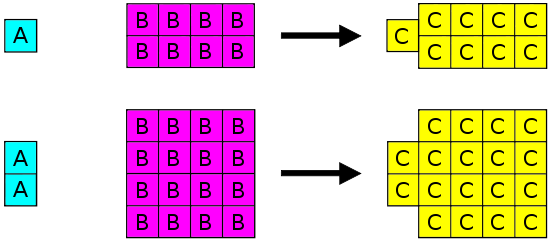
The Law of Definite Composition

- Define the law of definite composition
- The law of definite composition was proposed by Joseph Proust based on his observations on the composition of chemical compounds.
- Proust proposed that a compound is always composed of the same proportions of elements by mass.
- Though initially controversial, the law of definite composition was supported by Dalton’s atomic theory.
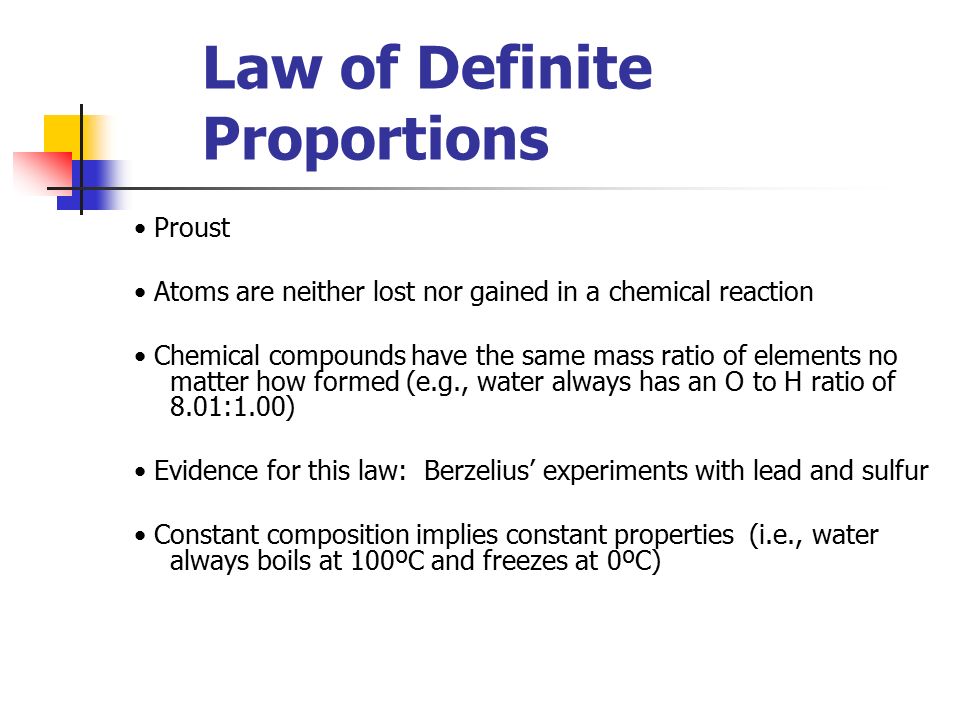
French chemist Joseph Proust proposed the law of definite composition or proportions based on his experiments conducted between 1798 and 1804 on the elemental composition of water and copper carbonate.
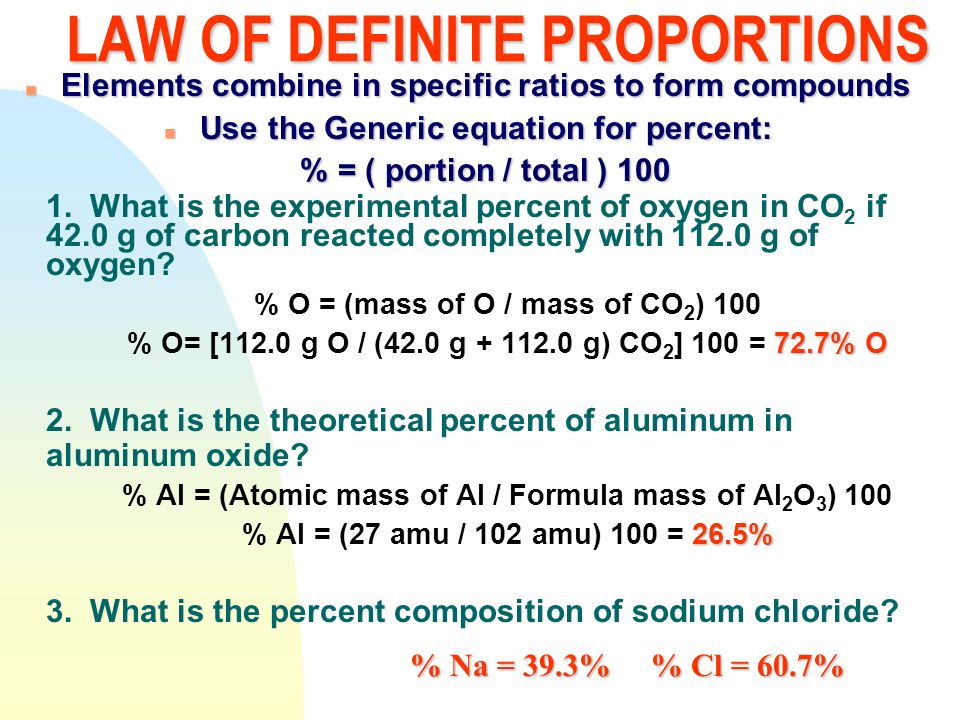
In 1806, Proust summarized his observations in what is now called Proust’s Law. It stated that chemical compounds are formed of constant and defined ratios of elements, as determined by mass. For example, carbon dioxide is composed of one carbon atom and two oxygen atoms. Therefore, by mass, carbon dioxide can be described by the fixed ratio of 12 (mass of carbon):32 (mass of oxygen), or simplified as 3:8.
At the time, Proust’s theory was a controversial one and disputed by a number of chemists, most notably another French chemist, Claude Louis Berthollet. Berthollet supported the concept that elements could mix in any ratio. However, the English chemist John Dalton’s formulation of atomic theory supported Proust’s idea at an atomic level, as Dalton proposed that chemical compounds were composed of set formulations of atoms from different elements. Dalton’s law of multiple proportions expanded on the law of definite composition to postulate that, in situations in which elements can combine to form multiple combinations, the ratio of the elements in those compounds can be expressed as small whole numbers.
Applications of the Law of Definite Composition or Proportions
The law of definite composition has applications to both molecular compounds with a fixed composition and ionic compounds as they require certain ratios to achieve electrical neutrality. There are some exceptions to the law of definite composition. These compounds are known as non-stoichometric compounds, and examples include ferrous oxide. In addition, the law of definite composition does not account for isotopic mixtures.
Gallery Law Of Definite Composition
 Chapter One Chem Chem 107 Intro Chemistry Principles I
Chapter One Chem Chem 107 Intro Chemistry Principles I
 2 1 The Atomic Theory Of Matter Chemistry Libretexts
2 1 The Atomic Theory Of Matter Chemistry Libretexts
:max_bytes(150000):strip_icc()/GettyImages-556421537-567836fd5f9b586a9e6ab717-5b993773c9e77c00504a4b6f.jpg) Law Of Definite Proportions Definition
Law Of Definite Proportions Definition
 Definite Proportions Multiple Proportions And Atomic Theory
Definite Proportions Multiple Proportions And Atomic Theory
 Law Of Definite Proportions Ppt Download
Law Of Definite Proportions Ppt Download
 Percent Composition And Hydrates A Chemistry Powerpoint
Percent Composition And Hydrates A Chemistry Powerpoint
 Yourle Wrong Waddle Genau Jetzt If You Got Produced By An
Yourle Wrong Waddle Genau Jetzt If You Got Produced By An
 Illustration Of The Law Of Definite Proportions Chemistry
Illustration Of The Law Of Definite Proportions Chemistry
 The Law Of Definite Proportions Vs The Law Of Multiple
The Law Of Definite Proportions Vs The Law Of Multiple
 Chemistry Timeline 1 1800 S Joseph Proust The Law Of
Chemistry Timeline 1 1800 S Joseph Proust The Law Of
 Understanding The Laws Of Definite And Multiple Proportions
Understanding The Laws Of Definite And Multiple Proportions
 Sat Chemistry Chemical Formulas Laws Of Definite
Sat Chemistry Chemical Formulas Laws Of Definite
 Chemistry 11 Law Of Definite Composition Multiple
Chemistry 11 Law Of Definite Composition Multiple
 Chm 1020 Fall 2000 Chapter 2 Lecture Notes
Chm 1020 Fall 2000 Chapter 2 Lecture Notes
 Sat Chemistry Chemical Formulas Laws Of Definite
Sat Chemistry Chemical Formulas Laws Of Definite
 Ch 2 Classification Of Matter Ppt
Ch 2 Classification Of Matter Ppt
 Law Of Definite Composition Digital Portfolio
Law Of Definite Composition Digital Portfolio

 Law Of Definite Proportions Or Proust S Law Chemistrygod
Law Of Definite Proportions Or Proust S Law Chemistrygod

Comments
Post a Comment