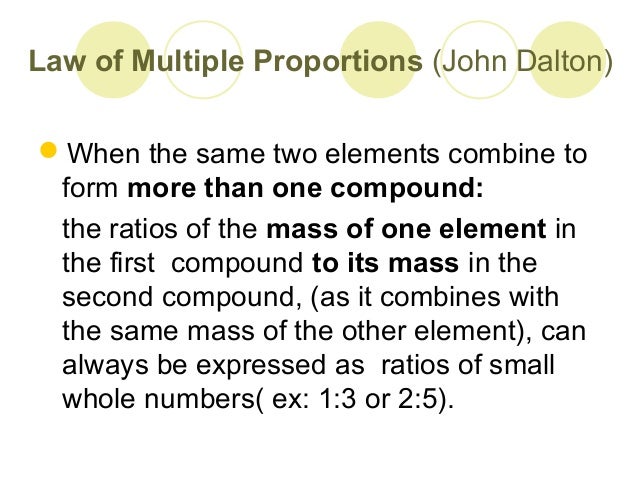
Understand the Law of Multiple Proportions and How It's Used
This is a worked example of a chemistry problem using the law of multiple proportions.
Two different compounds are formed by the elements carbon and oxygen. The first compound contains 42.9% by mass carbon and 57.1% by mass oxygen. The second compound contains 27.3% by mass carbon and 72.7% by mass oxygen. Show that the data are consistent with the law of multiple proportions.
Therefore, the masses of oxygen in the two compounds that combine with a fixed mass of carbon should be in a whole number ratio. In 100 grams of the first compound (100 is chosen to make calculations easier), there are 57.1 grams oxygen and 42.9 grams carbon. The mass of oxygen (O) per gram of carbon (C) is:
57.1 g O / 42.9 g C = 1.33 g O per g C
In the 100 grams of the second compound, there are 72.7 grams of oxygen (O) and 27.3 grams of carbon (C). The mass of oxygen per gram of carbon is:
72.7 g O / 27.3 g C = 2.66 g O per g C
Dividing the mass O per g C of the second (larger value) compound:
This means that the masses of oxygen that combine with carbon are in a 2:1 ratio. The whole-number ratio is consistent with the law of multiple proportions.
While the ratio in this example problem worked out to be exactly 2:1, it's more likely chemistry problems and real data will give you ratios that are close, but not whole numbers. If your ratio came out like 2.1:0.9, then you'd know to round to the nearest whole number and work from there. If you got a ratio more like 2.5:0.5, then you could be pretty certain you had the ratio wrong (or your experimental data was spectacularly bad, which happens too). While 2:1 or 3:2 ratios are most common, you could get 7:5, for example, or other unusual combinations.
The law works the same way when you work with compounds containing more than two elements. To make the calculation simple, choose a 100-gram sample (so you're dealing with percentages), and then divide the largest mass by the smallest mass. This isn't critically important—you can work with any of the numbers—but it helps to establish a pattern for solving this type of problem.
The ratio won't always be obvious. It takes practice to recognize ratios.
In the real world, the law of multiple proportions doesn't always hold. The bonds formed between atoms are more complex than what you learn about in a chemistry 101 class. Sometimes whole number ratios don't apply. In a classroom setting, you need to get whole numbers, but remember there may come a time when you'll get a pesky 0.5 in there (and it will be correct).
Gallery Law Of Multiple Proportions
 Law Of Multiple Proportions Need Method Check Please
Law Of Multiple Proportions Need Method Check Please
 Law Of Multiple Proportions Practice Problems Chemistry Examples Fundamental Chemical Laws
Law Of Multiple Proportions Practice Problems Chemistry Examples Fundamental Chemical Laws
 Understanding The Laws Of Definite And Multiple Proportions
Understanding The Laws Of Definite And Multiple Proportions
 Law Of Multiple Proportions And Law Of Definite Proportions
Law Of Multiple Proportions And Law Of Definite Proportions
 Solution Which Of The Following Sets Ill Chemistry
Solution Which Of The Following Sets Ill Chemistry
 Difference Between Law Of Definite Proportions And Law Of
Difference Between Law Of Definite Proportions And Law Of
 Law Of Multiple Proportions Definition Examples
Law Of Multiple Proportions Definition Examples
 Solved 3 34 Show That Each Of The Following Sets Of Data
Solved 3 34 Show That Each Of The Following Sets Of Data
 Mass Laws Law Of Conservation Of Mass Law Of Constant
Mass Laws Law Of Conservation Of Mass Law Of Constant
 Law Of Multiple Proportions Chemistry Encyclopedia
Law Of Multiple Proportions Chemistry Encyclopedia
 Law Of Multiple Proportions And Law Of Definite Proportions
Law Of Multiple Proportions And Law Of Definite Proportions
 Solved Show That These Results Are Consistent With The La
Solved Show That These Results Are Consistent With The La
 Synonyms For Law Of Multiple Proportions Thesaurus Net
Synonyms For Law Of Multiple Proportions Thesaurus Net
 Dalton S Law Of Multiple Proportions Illustration Learnodo
Dalton S Law Of Multiple Proportions Illustration Learnodo
 Ch 1 5 Law Of Multiple Proportions Daltons Atomic Theory
Ch 1 5 Law Of Multiple Proportions Daltons Atomic Theory
 Law Of Definite Proportions Law Of Multiple Proportions
Law Of Definite Proportions Law Of Multiple Proportions
 Law Of Multiple Proportions Definition Examples Video
Law Of Multiple Proportions Definition Examples Video
 Law Of Definite Composition And Law Of Multiple Proportions
Law Of Definite Composition And Law Of Multiple Proportions
 2 5 The Law Of Multiple Proportions And Dalton S Atomic
2 5 The Law Of Multiple Proportions And Dalton S Atomic
 The Law Of Definite Proportions Vs The Law Of Multiple
The Law Of Definite Proportions Vs The Law Of Multiple
 Law Of Multiple Proportions Definition Examples Video
Law Of Multiple Proportions Definition Examples Video
 Multiple Proportions Some Extra Questions
Multiple Proportions Some Extra Questions
 An Experiment To Illustrate The Law Of Multiple Proportions
An Experiment To Illustrate The Law Of Multiple Proportions
 State And Explain The Law Of Multiple Proportions How Is The Law Of Multiple Proportions Different 2
State And Explain The Law Of Multiple Proportions How Is The Law Of Multiple Proportions Different 2
 Law Of Multiple Proportions Chemistrygod
Law Of Multiple Proportions Chemistrygod
 What Is Law Of Conservation Of Mass Law Of Constant
What Is Law Of Conservation Of Mass Law Of Constant
Comments
Post a Comment